

Living with CIDP? You Are Not Alone
Who Can Participate in the Study?
Participants must:
- Be an adult aged 18 years or older.
- Be diagnosed with active CIDP.
- Additional eligibility criteria will be assessed by the study doctor or staff during the screening process prior to being enrolled in the study and again before receiving any investigational medicine.
Participants must not:
- Be diagnosed only with pure sensory type CIDP or chronic immune sensory polyradiculopathy (CISP).
- Test positive for Hepatitis B, Hepatitis C, or human immunodeficiency virus (HIV).
- Have a current or recent history (within the last 12 months) of drug abuse or alcoholism.
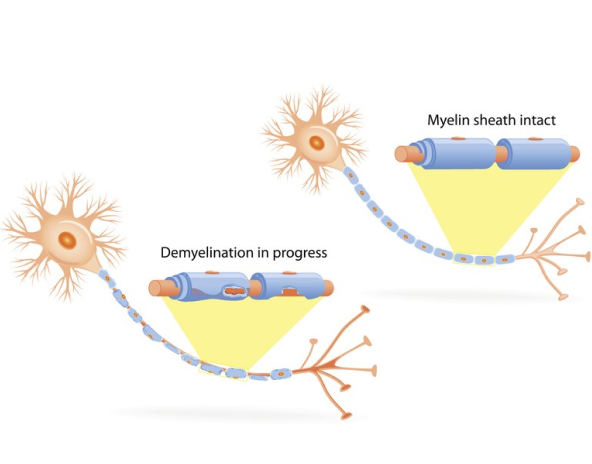
What is CIDP?
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare disease where the body’s natural defenses, like antibodies, attack the covering of the nerves, called myelin, and damage nerve function. While symptoms aren’t the same for everyone, CIDP can make your arms and legs feel weak, and slow your movements.
CIDP can affect people differently. Symptoms can worsen over time for some, while others may have symptoms that stabilize and then return or relapse. Other people may experience a CIDP episode that lasts for several years.
About the Study
Researchers are looking for new ways to treat CIDP. In the ARISE study, researchers want to evaluate the effectiveness and safety of an investigational medication in adults with active CIDP.
The main goal of the study is to learn whether an investigational medication is safe and effective in delaying relapse in adults with chronic inflammatory demyelinating polyneuropathy (CIDP). Eligible participants with active CIDP may be enrolled. Patients who are receiving standard CIDP medications as well as those not on any treatment may be eligible to participate.
Depending on medications a patient is taking, participants may be requested to either discontinue or slowly reduce the dose of medications. Enrolled participants will be closely monitored by a neuromuscular neurologist throughout the study.
The ARISE study aims to include around 201 participants across the globe. Participation in this study may help to advance CIDP research and identify future potential treatments.
It’s important to know that the safety and efficacy of the investigation medication have not yet been established by the FDA.
Please speak to your regular doctor to learn more about the trial and enrolling locations and if this is something you may be able to participate in.
What will Happen in this Study
If you are interested in joining this study, you will need to visit a nearby study site. A study doctor will explain the study to you. If you agree to join, the study doctor and other site staff will give you a medical exam to confirm your CIDP diagnosis and ask you questions about your CIDP symptoms, your medical history, and the medications you are taking to make sure you are eligible to take part in the study. If you qualify for all portions of the study and wish to continue, you can expect to spend up to 2–5 years in the study. Your participation is voluntary and you may stop the study at any time. Participants may be reimbursed for study-required travel.

The study will happen in several stages:
Screening/Run-in:
Participants will have their CIDP diagnosis confirmed through medical and neurological examinations during the screening phase. Those who are currently being treated for CIDP may be asked to discontinue or slowly reduce medications, also known as a run-in period. During this period, participants will be closely monitored by the study doctor to confirm their CIDP is active and not in remission.
Open-Label Phase:
Participants who qualify will receive the investigational medication through an IV infusion every two weeks. The goal of this phase is to see which participants respond to the investigational medication.
Double-Blind Phase:
Participants who respond to the investigational medication in the Open-Label Phase may be eligible to continue to this step. Eligible participants will be randomly assigned (by chance such as the flip of a coin) to either:
- Continue to receive the investigational medication through an IV infusion every two weeks;
- OR
- Receive placebo (a treatment that looks exactly like the investigational medication but has no active ingredients) every two weeks.
During this phase of the study, none of the participants, study doctors, or staff will know which study treatment each participant is getting.
Open-Label Phase:
The study doctor will determine if you are eligible to enter this phase, if you are interested in continuing. All eligible participants will have the opportunity to receive the investigational medication during this portion of the study.

Those who join the study will:
- Receive a thorough medical and neurological exam to confirm study eligibility, including confirming CIDP diagnosis.
- Receive the investigational medication via IV infusion for a portion of the study.
- Receive study-related medical care and the investigational medication at no cost.
In between infusion weeks, you will have phone calls with the study site to check in on how you are doing. If you qualify for all portions of the study and wish to continue, your participation may last up to 2-5 years.

During study site visits, the study doctor will:

- Give you a physical examination and neurological examination
- Check your heart health using a test called “electrocardiogram” (ECG)
- Collect blood and urine samples
- Check your CIDP symptoms and how you are feeling
- Ask about any new medications and/or vaccinations
- Ask about any new or worsening medical problems you might be having
What You Can Do Next
- Talk to your treating physician to verify that you have CIDP and discuss the study. For more information, you can view and share the study listing on ClinicalTrials.gov: https://clinicaltrials.gov/study/NCT05327114.
- Complete the pre-screener questionnaire on this website by clicking on the “See if You Pre-Qualify” button.
- If you qualify, you will be asked to fill out your information on the intake form and agree to the Information Release Consent so that the nearby study site you select may contact you.
- If you agree, your contact information will be shared with a nearby study site. Please note that your personal information will not be shared with Janssen Research & Development, LLC (the company sponsor for this clinical study), or any third party not involved in the clinical study.
- A study site team member will reach out to schedule a phone call to those who express interest to provide more information on the study and your potential participation.
Study Locations
Talk to your doctor if you are interested in learning more but don’t see a study site near you.
See if You are Eligible
Resources
Whether you or a loved one is affected by CIDP, you are not alone. Use the resources below to find information and connection through organizations dedicated to supporting individuals and families with CIDP.
| Name | About | Phone | Website |
|---|---|---|---|
| GBS-CIDP Foundation | The GBS-CIDP Foundation is a global nonprofit organization supporting individuals and their families affected by Guillain-Barre’ syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), Multifocal Motor Neuropathy (MMN) and related conditions through a commitment to support, education, research and advocacy. | 866-224-3301 | https://www.gbs-cidp.org/ |
| National Organization for Rare Disorders (NORD) | NORD’s mission is focused on improving the health and well-being of people with rare diseases by driving advances in care, research, and policy. | 617-249-7300 | https://rarediseases.org/ |
| The Foundation of Peripheral Neuropathy | The Foundation of Peripheral Neuropathy is committed to improving the lives of those affected by peripheral neuropathy through awareness, education, advocacy, and research to advance the discovery of new therapies and cures. | 877-883-9942 | https://www.foundationforpn.org/ |
| The Autoimmune Association | The Autoimmune Association is a nonprofit organization dedicated to increasing and improving autoimmune awareness, advocacy, education, and research. | 586-776-3900 | https://autoimmune.org/ |
